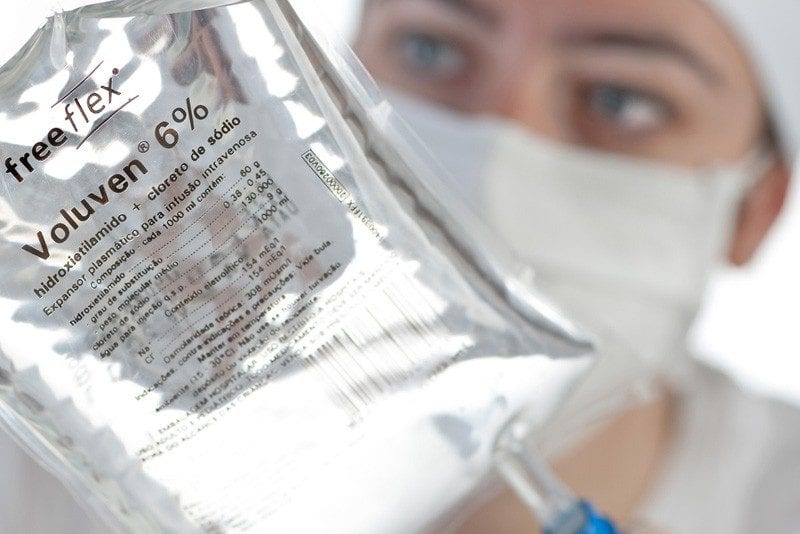
NEWCASTLE, AUSTRALIA – The end seemed nigh for Voluven. The volume expander has been on the ropes after large clinical trials demonstrated increased risks of acute kidney injury and need for dialysis following its use in critically ill patients. In addition to being contraindicated in burns, liver disease, trauma, intracranial haemorrhage, coagulopathy, and sepsis, the hapless colloid appeared to be a touch on the lethal side in the sort of sick, the vaguely unwell, and the pretty-good-thanks-but-got-a-touch-of-the-sniffles population.
That was until groundbreaking research released today in the Hunter Valley of NSW. An analysis of 4,379 axillary rolls placed over a 9-month period in a 15 theatre complex, heralded an unlikely comeback for the Fresenius-Kabi flagship. In a head-to-head trial with traditional stalwarts Hartmanns solution and a rolled-up Newcastle Herald, the long-suffering starch came up trumps, with a significantly lower incidence of post-op skin necrosis and a vanishingly low number of brachial plexus injuries. (p = 0.001).
HNELHD head of Anaesthetic Research, Prof J Dimbleby explains:
“We aren’t really sure what’s going on but it just seems to work. In all honesty we were trying to figure out what the hell to do with the 8,500 or so bags of Voluven sitting around in storage waiting to expire, so we thought we’d give it a try,” said the clearly-surprised scientist.
“The really amazing thing was that we came up with a use for the white port. You know, the one with the little label that says, ‘Under no circumstances use this port’? We figured that couldn’t be right. Turns out it is incredibly robust and good for forcibly removing the bag under the weight of a 250-kg. patient. And let’s face it, we have no shortage of nutritional over-achievers in this part of the world.”
New studies are looking at also using Voluven on the labor deck for left lateral displacement of pregnant patients and as a wrist extender for radial arterial line placement.